We’ve all heard that the human body is roughly 60% water.[1]
But where does that water actually go? Where is it stored? The answer is, in your cells, which consist of an even greater proportion of water by weight: Your cells are actually 70% water![2]
What Does It Mean To Be Hydrated?
Arguably, the predominance of water in the cell illustrates further the importance of staying hydrated. Ultimately, everything your body does is a function of cellular metabolism, which depends on water to function properly.

Hydration: The Ultimate Guide. How do you stay hydrated? This article covers the best approach to fluid and electrolyte intake, also cautioning against what may harm your hydration status
Your cells rely on water to act as a solvent for certain nutrients. Water soluble nutrients get their name from the fact they dissolve in water,[3] thus creating a nutrient-rich solution which is then transported throughout your body, to cells, and within your cells, from organelle to organelle. The passive transport of nutrients across cellular membranes, through mechanisms like osmosis and diffusion, depends on the movement of water along with the nutrients to maintain osmotic balance and concentration gradients.[4]
How Sodium Can Illustrate What It Means To Be Properly Hydrated
Consider sodium.
Here, the difference between bodily hydration and cellular hydration is thrown into sharp relief. You can drink all the water you want, but if you’re not also supplying your body with the nutrients and solutes it needs to maintain osmotic equilibrium, you can get into big trouble.
If you go out and train really hard, you’ll sweat a lot. And we all know that sweat is made up of water,[5] so we have to drink water to replace what’s lost in sweat.
But we also all know that sweat is salty. So what happens if you replace the water you lose, but not the sodium? In extreme cases, this causes a condition called water intoxication that’s characterized by your cells’ inability to self-regulate through osmotic mechanisms, thanks to an imbalance of solutes.[6]
In order to gain some insight into the molecular mechanism behind water intoxication, we only need to consider that this condition is also called hyponatremia – a Greek word that literally translates to low sodium.
Water naturally moves from an area of low solute concentration to high solute concentration, so when there’s too little sodium outside your cells as a result of excessive sweating, water starts moving into your cells. If sodium levels are low enough, this process is essentially uncontrolled, meaning that your cells start to swell from the extra water volume. This leads to edema, particularly in the brain, which can damage tissue and cause death.[7]
What happens during chronic hyponatremia
Hyponatremia can certainly turn into a life-threatening emergency, but don’t assume that it will always present such dramatic symptoms. Hyponatremia can be chronic and asymptomatic for years.
Your body has mechanisms it can use to cope with chronic hyponatremia, the most problematic of which is arguably bone resorption. Bone tissue contains a lot of sodium, so if your body needs sodium, it’ll start breaking down that bone to get at the sodium. If this process goes on long enough, it can lead to osteoporosis.[8] (We’ll look at other ways of avoiding bone resorption and hypercalcemia later in this article.)
In other words, proper hydration is a lifelong endeavor, requiring daily effort. Just because you’re not presenting symptoms of acute hyponatremia doesn’t mean you’re properly hydrated. You need to supply your body not only with enough water, but also with enough sodium to maintain proper electrolyte balance.
The question of low sodium intakes
It may surprise you to read the above passage, especially since public health messaging since the mid-20th century has insisted that a lower sodium intake is always better. Perhaps you also think that, in practice, it’s not really possible to develop a chronic sodium deficiency – that the daily requirement for sodium is low enough we don’t need to worry about getting enough from our diet.


True salt — as in sodium chloride — is white. Pink salts may contain other minerals, such is iron oxide (rust), which may not be optimal for health if one is already on iron overload.
Recent research is challenging the assumption that sodium restriction is always good. Ironically, it turns out that the consequences of consuming too little sodium are very similar to those of consuming too much. In fact, current evidence has demonstrated a J-shaped relationship between sodium intake and cardiovascular events. A 2015 research review, looking at studies with over 300,00 combined participants, found that the lowest risk of cardiovascular events and deaths occurs in populations with a sodium intake between 3 and 5 grams per day.[9]
So consuming less than 3,000 mg of sodium per day can actually increase one’s risk of cardiovascular disease and cardiovascular events. Note that the current recommended maximum daily sodium intake is 2,300 mg – well below the the intake range identified as conferring the lowest mortality risk!
This is corroborated by other research, such as a 2011 study, published by the Journal of American Medicine, that had over 28,000 participants and ultimately concluded that sodium intakes below 3,000 mg/day increased risk of being hospitalized for congestive heart failure.[10]
So, you probably need more salt than you think – and at a minimum, if you’re sweating a lot, it’s probably not a great idea to drink a bunch of water without also replenishing your body’s store of electrolytes.
Sodium is Not the Only Electrolyte
The interplay between sodium and water is an instructive illustration of what it really means to be hydrated, but even sodium is only one part of a much bigger story.


This paper’s title is so spectacular that it deserves its own call-out.[11] So what gives? We simultaneously believe that sodium recommendations can go higher, but potassium does need to be supplemented in modern dieters as well.
For one thing, sodium needs to be balanced with potassium, another important electrolyte mineral. So not only do you need to consume sodium along with water, you also need to balance the sodium you consume with potassium for the best hydration and cardiovascular outcome.[12]
The picture gets even more complicated when you consider the fact that in addition to sodium and potassium, there are additional electrolytes we must consider as part of an optimal hydration balance – namely chloride, magnesium, calcium, phosphate, and bicarbonates.[12] In order to properly hydrate, one must consume all of these electrolytes, and in the correct proportions to suit your biology and lifestyle. Too much or too little of any one of these can lead to suboptimal hydration status, thus compromising both mental and physical performance.
The Hydration Spectrum – Everyday Implications
Even slight differences in hydration status can have major consequences for everyday health and performance. For example, a 2012 research review on hydration found that being dehydrated by even just 2% of total body water volume can significantly impair performance on tasks requiring attention, psychomotor, and immediate memory skills.[13]
For the average person — but especially for white collar workers and anyone whose work is primarily cognitive in nature — maintaining optimal hydration is a hugely important and ongoing task. In order to perform at our best at the things we do to earn a living and survive, we must constantly replenish lost water, even if we’re sedentary. And that means we must also, at the same time, replace electrolytes in order to prevent osmotic balance from being thrown out of whack.
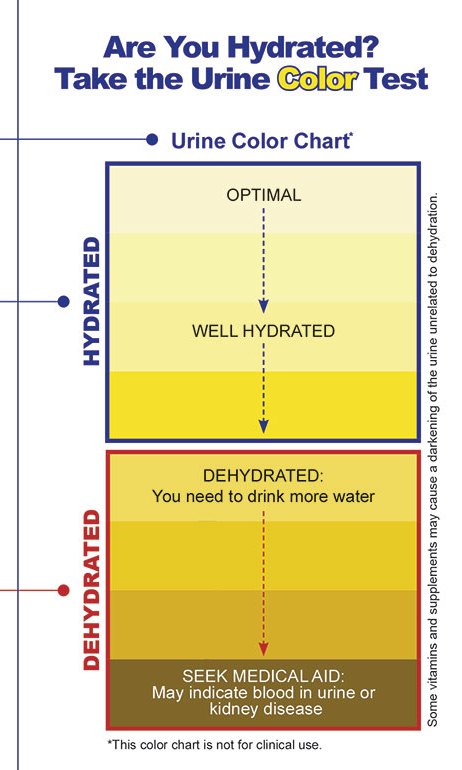

Here’s an image you’ll find posted inside various US Military bathrooms for people to check on their hydration levels. Note that they do not recommend clear urine!
To make things even more complicated, there are a lot of highly variable factors that can potentially affect an individual’s hydration status, in addition to water and electrolyte requirements:
- Fluid intake
- Fluid loss (through sweating, breathing, urination, etc.)
- Climate and environment – low humidity and high altitude environments increase rate of fluid loss
- Physical activity – the more active you are, the more fluid and electrolytes you lose
- Metabolic rate – the harder your cells are working, the more water you need to provide
- Medical conditions (e.g. diabetes, kidney disease)
- Alcohol and caffeine consumption – both diuretics that increase fluid loss
- Individual differences in height, weight, age, genetics, etc.
How To Hydrate
So with all of that in mind, how exactly can we ensure that we are and remain optimally hydrated? What are best hydration practices?
Water intake estimations and theories
You may be surprised to learn that when it comes to baseline fluid requirements, there isn’t yet a definitive answer to this seemingly basic question. A 2012 research review that analyzed five of the most commonly-used equations for calculating individual fluid requirements found that each formula produced a significantly different result, with a pretty wide range. Using a hypothetical 75-year-old, 175-cm-tall man weighing 127 kg as an example, the authors of the review found that their five selected equations recommended anywhere from 3,810 mL (128 ounces) to 1,999 mL (67 ounces) daily water intake.[14]
That’s a huge range! What’s right? It’s tough to know, and it gets tougher as body sizes become more extreme.
To estimate the ounces of water you should drink daily, PricePlow prefers to use an equation proposed by the University of Missouri, which is effective and very simple: multiply your body weight (in pounds) by 0.5.[15] So in the reviewer’s example above, that 127 kg (280 lb) man would instead need 140 ounces of water per day, but an average 175 pound man would need 87.5 ounces.[15]
We think that’s reasonable – for larger individuals, it’s on the higher side of the range quoted by the review, but that’s not necessarily a bad thing, and the estimate is closer for most others. Remember, even slight dehydration can cause significant impairments to physical and mental performance. It’s better to be safe than sorry, and consume a little extra fluid, so long as you also match it with a balanced electrolyte intake.
Adding more for exercise time
When it comes to factoring in the impact of physical exercise, there’s a useful rule of thumb – just add 12 ounces of water for every 30 minutes of exercise.[15]
However, if you want to be more precise, the American College of Sports Medicine has some extremely specific and up-to-date recommendations:[16]
- 5 to 7 mL/kg of body mass 4 hours prior to exercise
- Enough fluid to reduce body mass changes to less than 2% during activity
- 450 to 675 mL for every 0.5 kg of body mass lost during exercise
Even if you don’t manage to hit targets 1 and 2, you should definitely be able to hit no. 3. In practice, this works out to drinking 15 to 23 ounces of water for every 1.1 pounds of body weight lost during exercise.[16]
Obviously, the more competitive you are, the more you’ll want to dial into this – 12 ounces for every 30 minutes of exercise will do well for the majority of readers. Serious competitors will want to dial in more accurately.
Electrolyte losses during exercise
So, now that we’ve got the water side of things covered, we need to think about electrolyte losses and replenishment pertaining to exercise.


We only needed a little bit of hydration support after this one… (Ben and Mike rolled in Brian’s Jiu-Jitsu class the night before Episode #111 of the PricePlow Podcast was filmed with Brian Littlefield)
The first fact to consider is that sodium accounts for the vast majority of electrolytes lost in sweat. Data shows that the human body loses roughly 0.9 grams of sodium per liter of sweat – the next biggest electrolyte loss is potassium at a mere 0.2 grams per liter.[5]
If you consider the fact that sweat rates during exercise are generally somewhere between 0.5 and 2.0 liters per hour,[17] and that the average rate of sweating in athletes is roughly 1.2 liters per hour,[18] we think it’s a good rule of thumb to assume you’ll lose 1 liter of sweat per hour of exercise – which translates to 900 mg of sodium lost per hour of exercise.
So if you’re doing 20-30 minutes of HIIT, you’ll probably want to rehydrate with somewhere between 200 and 400 mg of sodium.
Remember, the 3-5 gram daily sodium (not salt!) intake we discussed earlier is based on a population average, not athletes. So if you’re sweating for an hour a day, your sodium requirement for optimized cardiovascular risk could be closer to 4 grams than 3. As a performance-centric group, PricePlow frequently cites Valentine’s 2007 article, “The Importance of Salt in the Athlete’s Diet”, for this very reason.[19]
A quick guide to shopping for hydration supplements
In general, our recommendation for hydration supplements depend on your diet and activity level:
- Athletes who work very hard and sweat a lot can skew towards higher-sodium, moderate potassium products.
- More sedentary consumers who eat processed food or the SAD “Standard American Diet” are wise to consume higher potassium, lower sodium drinks.
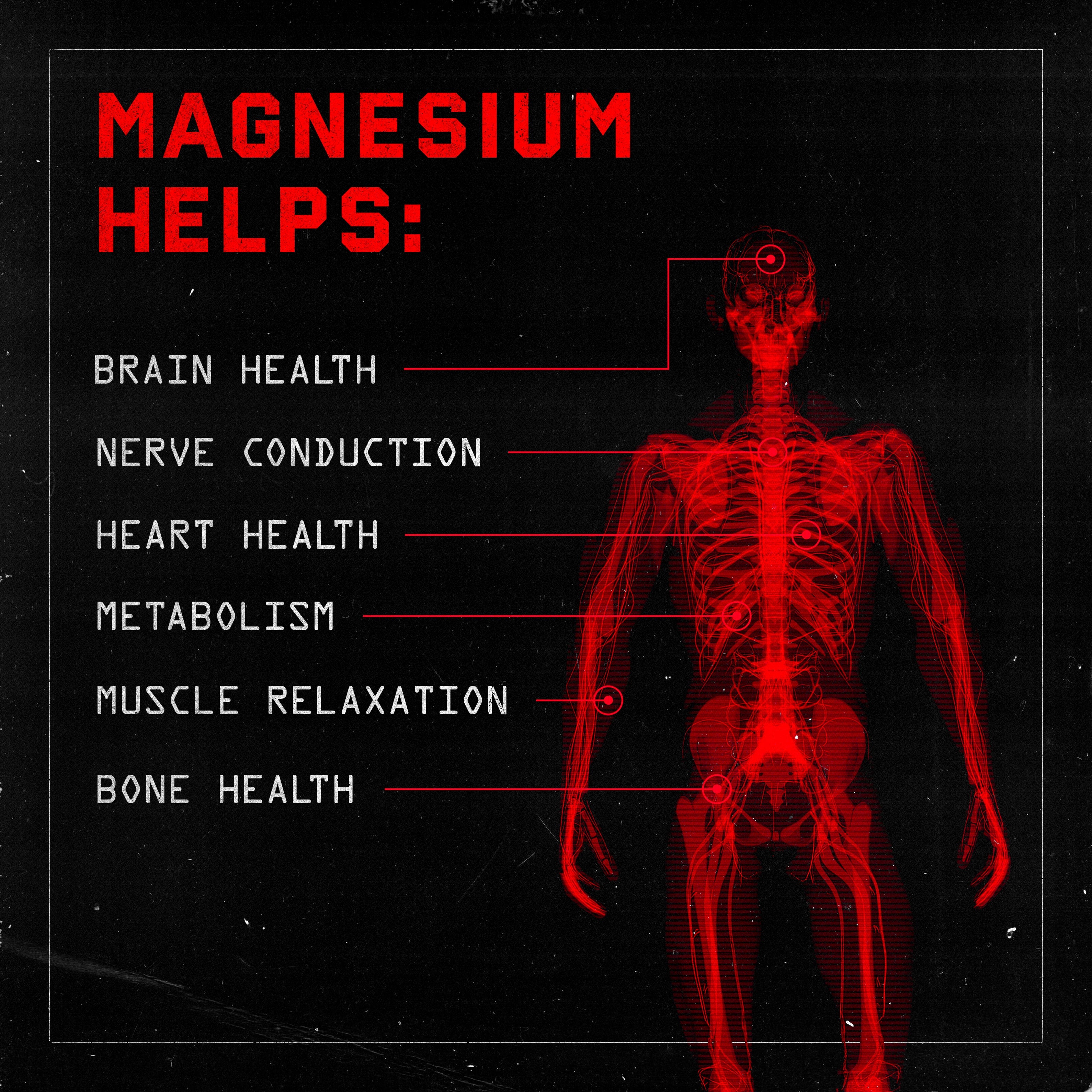
- Practically everyone needs more magnesium, which we appreciate both supplementally and topically (through magnesium chloride baths and lotions).
We’re not here to make recommendations about exactly how much sodium you, the reader, should consume on a daily basis. For that, you should talk to your doctor and precisely track your diet for a few weeks.
But we do want to make the point that for proper hydration, you should choose a hydration formula that includes a significant amount of sodium, as well as all the other electrolyte minerals.
Potassium and magnesium are the underrated components
Most of us get enough calcium, but potassium and magnesium — the last two of the four major electrolyte minerals — are known to be shortfall nutrients in the American diet, meaning most Americans don’t get enough of either.[20-22] The situation with magnesium is particularly bad – half of American adults don’t consume enough magnesium![21,22] The population-wide magnesium deficiency is especially devastating, given the crucial role mineral plays in hundreds of different metabolic processes.
We’ll leave you with this mostly-unknown bit of information regarding potassium: a 2019 study looking at potassium consumption in the treatment of obesity/metabolic syndrome noted that “the strongest correlate of the decline in BMI was the increase in dietary potassium intake”.[23] Don’t ignore these minerals!
But we also need to beware of what may strip the minerals from your bones and body:
Beware of toxicities that can impair hydration
Finally, we’ve been coming into more research concerning certain vitamins — especially the fat-soluble / fat-storable alcohols — which are prone to toxicity as they accumulate in the system and cause severe mineral status problems.
The following research may be of interest to those of you who are into “mega-dosing” certain vitamins:
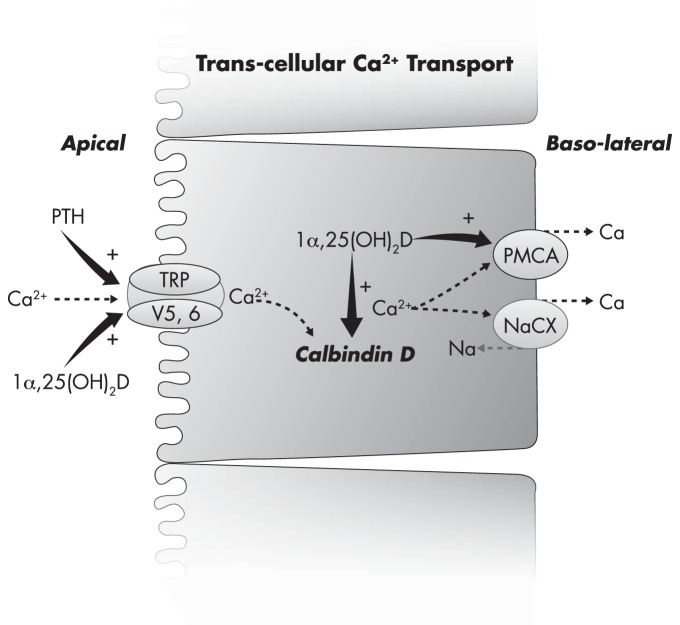

Could excess vitamin D3 be assisting in the extrusion of calcium from within the cell into the extracellular fluid, leading to hypercalcemia?![24] And can we be sure that adding Vitamin K is enough to prevent it with 100% success rate?
- Excess Vitamin A (retinol) has been shown to promote bone resorption and hypercalcemia,[25-29] and potentially even cardiovascular calcification.[30]
- Excess Vitamin D3 (cholecalciferol) is also known to cause hypercalcemia.[24,31]
- Vitamin A’s active metabolite, retinoic acid, blocks cellular potassium channels.[32]
- Vitamin D may induce renal potassium-wasting.[33]
- Vitamin A metabolite, retinoic acid, may also deplete magnesium in cells.[34]
To address the calcification issue from vitamin D, most professionals who understand this pair it with vitamin K,[35,36] notably the only fat-soluble vitamin that’s not an alcohol.
To further complicate matters, vitamins A and D antagonize one another,[37] and vitamin A metabolites may also deplete vitamin K levels![38,39]
For these reasons, we’re beginning to ask ourselves if we’re truly deficient in natural vitamin D, calcium, magnesium, and potassium… or if we’re really just becoming toxic in their antagonists, namely the vitamin A class of molecules (retinoids).
This is why we’re beginning to express caution and concern about mega-dosing any fat soluble classes of alcohols like vitamins A & D, since they can accumulate and lead to subclinical problems that require evermore mineral supplementation. When it comes to these molecules, venturing too far from the RDA may not be the best idea.
Finally, we have to wonder out loud why one of the most popular hydration drinks on the market has so much vitamin A inside, when it seemingly does nothing positive for hydration, but may in fact antagonize what we need for appropriate electrolyte balance.
There’s got to be a better way:
Jocko Hydrate RTD – The Total Package
And for that, Jocko Hydrate RTD definitely fits the bill.
3:2:1 Sodium:Potassium:Magnesium Ratio
This RTD hydration product comes in 473 mL (16 fluid ounce) servings, each with 300 mg of sodium and 200 mg of potassium, plus 100 mg of magnesium, which we think is super critical – the most enlightened hydration formulas these days all contain a sizable amount of magnesium.
We’re big fans of this 3:2:1 ratio, which is a reasonable approach for most athletes. Those who are sweating gallons will want to find more sodium in their day, but those who are drinking potassium-only hydration beverages (that are a bit less… serious) will find themselves enjoying the moderate dose of sodium.
Don’t miss the zinc!
Where Jocko Hydrate really sets itself apart is that it contains 2 mg (25% DV) of zinc, which is a sign that this formula is geared toward serious athletes and other very active persons. Although it’s not discussed much, athletes actually have significantly increased requirements for zinc, as evinced by the fact that athletes generally have lower serum zinc levels than the population average, despite actually consuming more zinc than average.[40]
Despite the fact that athletes often struggle to get enough zinc, it’s especially important for male athletes because zinc is the master mineral of the male endocrine system. Research shows a strong positive correlation between serum zinc levels and serum testosterone levels,[41] and even mild zinc deficiencies can create major testosterone deficits.[42] Zinc also plays a key role in converting testosterone to dihydrotestosterone (DHT),[43] the most androgenic form of testosterone that produces the masculinizing effects typically associated with this androgen.
Obviously, testosterone is hugely important for performance, recovery, and muscle growth – and yes, we do lose significant amounts of zinc in sweat, to the tune of 4% of the RDA per hour of exercise.[44] That may not sound like a lot, but it can definitely add up, especially if you have borderline zinc status to begin with.
The added vitamins – making a homemade “ZMA” style stack!
Finally, Jocko Hydrate contains vitamin B6, which is part of the traditional ZMA stack (along with zinc and magnesium), and the methylcobalamin form of B12, which is great for energy and metabolism, especially for those who don’t eat much meat.
If you’ve got your own water and want to add your own hydration, you can also see Jocko Hydrate sticks, which has even greater doses of the minerals discussed in this article.
Conclusion: Hydration depends on your diet and activity
We wish we could give you a “one size fits all” solution to hydration, but it’s not even close to that easy. Not only do your needs depend on your existing diet and activity levels, they can also be affected by your other supplements and nutrient intakes! This is not as simple as anyone wants to make it out to be, and to do it right, it requires testing, tracking, and time.
Finally, we’d be remiss if we didn’t quickly mention a few additional ingredients that function as osmolytes, maintaining osmotic balance and pressure in cells. Most of our readers know them well in betaine (trimethylglycine), glycerol, and taurine, which you’ll find in sports nutrition pre-workout supplement formulas all over the market.
In general, we want to see sodium, potassium, and magnesium all together. When they’re combined, ratios are less likely to get heavily out of skew, regardless of who you are. But as you get more serious about your athleticism, you’re going to want to really start dialing into the calorie counter app to see where you’re at, remove as many anti-nutrients as possible, and slowly change one variable at a time as you continue your quest for peak performance.
This article was sponsored by Jocko Fuel, in preparation for the new Jocko Hydrate RTD launch. You can also find a full dose of betaine in Jocko Pre-Workout, for further athletic hydration. Thank you to Team Jocko for allowing us to put this resource together, and for providing us with excellent, naturally-sweetened formulas. You can sign up for PricePlow’s Jocko Fuel news alerts below:
Jocko Fuel – Deals and Price Drop Alerts
Get Price Alerts
No spam, no scams.
Disclosure: PricePlow relies on pricing from stores with which we have a business relationship. We work hard to keep pricing current, but you may find a better offer.
Posts are sponsored in part by the retailers and/or brands listed on this page.